

was performed by the researchers for treatment of genetic disorder ADA-SCID.
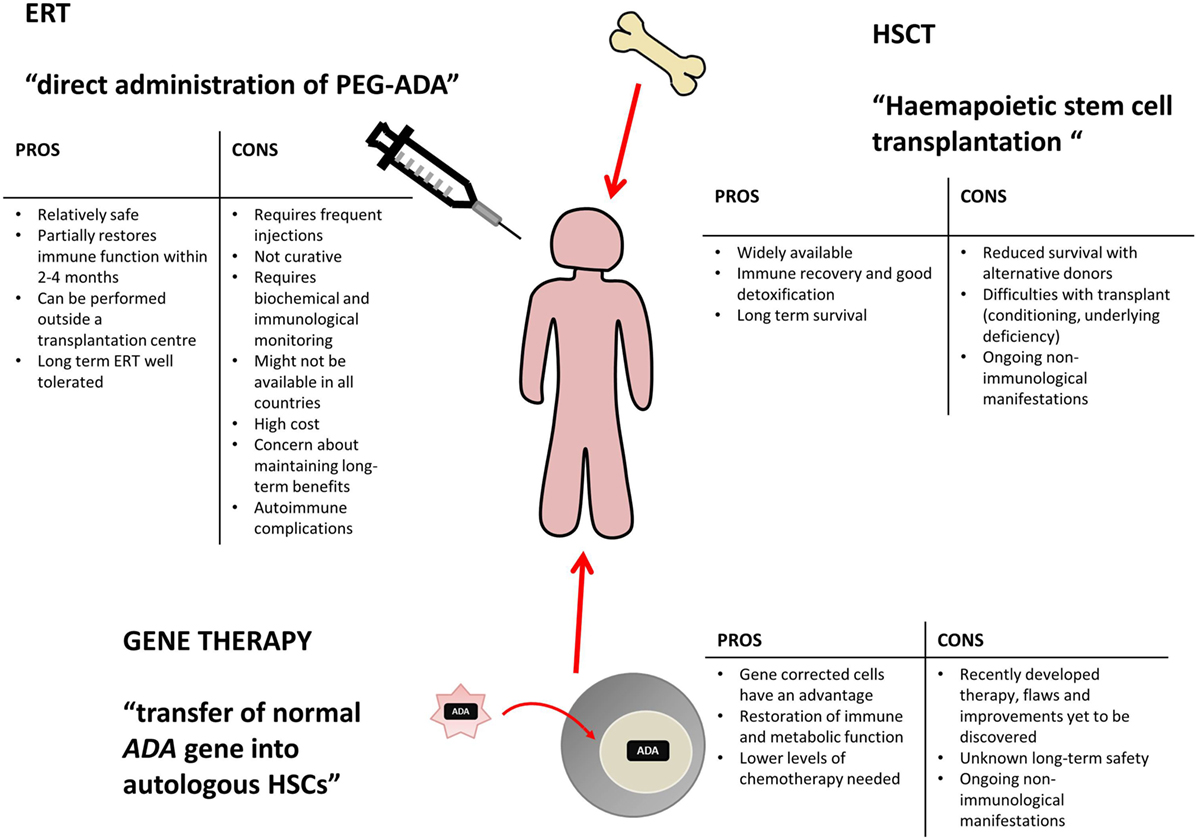
A retrovirus, which is capable of transferring its DNA into normal eukaryotic cells ( transfection ), is engineered to contain the normal human ADA gene. Based on encouraging preclinical proof of concept, gene editing for SCID-X1 may also soon find clinical application. Gene therapy is technique of recombinant DNA technology which involves the. SCID Gene Therapy Somatic Therapy for SCID Severe Combined Immunodeficiency Disease ( SCID ) is due to a defective gene for Adenosine Deaminase ( ADA ). Gene editing on the other hand could be used to correct a mutation in situ, or insert a working copy of the gene into the natural locus, allowing endogenous control of expression. Finally, all the current trials use integrating vectors as a “gene addition” approach, which predisposes not only to oncogenesis but also fails to completely recapitulate natural gene expression patterns. Using nontoxic conditioning, for example, antibodies that deplete HSPCs, before gene therapy would further improve the safety profile of this treatment. Since the initial trials of gene therapy for ADA-deficient SCID, almost 400 clinical trials of gene therapy have been performed for a broad array of conditions (cancer, cardiovascular disease. In addition to potential long-term toxicities such as impairment of linear growth, infertility, and school function problems, myelodysplastic changes may occur in the marrow and have been reported after autologous gene therapy for other disorders. Table I Historical and current trials of gene therapy for SCID-X1 VectorĪlthough the busulfan dose used in current trials is low, approximately one-third of the myeloablative dose, exposing these young infants to alkylating agents is not without consequences. Gene Therapy for ADA-SCID Shows Promise Research findings recently published in the New England Journal of Medicine provide hope that gene therapy may one day be an accepted treatment for ADA-SCID instead of an experimental one. NIAID researchers are using a novel gene therapy approach to successfully treat older children and young adults. This therapy has been tried in a small number of patients with varying degrees of success.

A modified γ-retrovirus vector for X-linked severe combined immunodeficiency. After infecting the cells, the virus’ work is done, and it dies. The approval of ADA-SCID gene therapy is the result of a joint effort among different stakeholders and exemplifies how the open cooperation between academic. A carrier molecule known as a vector ferries the normal gene into the patient’s blood-producing stem cells. Fondazione Telethon has recently learned that a patient suffering from a rare immunodeficiency of genetic origin, ADA-SCID, and treated in 2016 with gene. The process provides patients with the normal gene they lack. Hacein-Bey-Abina S, Pai SY, Gaspar HB, Armant M, Berry CC, Blanche S, Bleesing J, Blondeau J, de Boer H, Buckland KF, Caccavelli L, Cros G, De Oliveira S, Fernández KS, Guo D, Harris CE, Hopkins G, Lehmann LE, Lim A, London WB, van der Loo JC, Malani N, Male F, Malik P, Marinovic MA, McNicol AM, Moshous D, Neven B, Oleastro M, Picard C, Ritz J, Rivat C, Schambach A, Shaw KL, Sherman EA, Silberstein LE, Six E, Touzot F, Tsytsykova A, Xu-Bayford J, Baum C, Bushman FD, Fischer A, Kohn DB, Filipovich AH, Notarangelo LD, Cavazzana M, Williams DA, Thrasher AJ. To find a safer and more effective process for curing children with SCID, scientists turned to gene therapy. Why Should I Register and Submit Results?.


 0 kommentar(er)
0 kommentar(er)
